SOPHiA DDM™ for
Solid Tumors
Translating complex data into insights
Clinical researchers are faced with the challenge of analyzing and interpreting increasingly complex genomic datasets. We help by generating insights from next-generation sequencing (NGS) data.
Streamline your workflow
Our genomic applications streamline the interpretation of complex genomic variants by combining a targeted, capture-based workflow with the analytical performance and advanced features of the SOPHiA DDM™ Platform.

Easy library preparation and capture kit
- Tailored probes for high on-target rate and coverage uniformity
- Ready-to-sequence in only 1.5 days
- Optimized multiplexing for a cost-effective process
- Compatible with various sequencers
Advanced analysis with SOPHiA DDM™ Platform
- Variant detection, annotation, and classification
- Easy visualization, filtering and reporting
- Secure storage of anonymized data
- Access to the latest scientific evidence on relevant alterations and to one of the largest networks of connected healthcare institutions
Accurate variant detection
Our SOPHiA DDM™ for Solid Tumors solutions enable advanced, in-house analysis of complex variant classes. The algorithm-powered capabilities of the SOPHiA DDM™ Platform support accurate detection of single nucleotide variants (SNVs), insertions and deletions (Indels) and copy number variations (CNVs), as well as mutational biomarkers, such as:
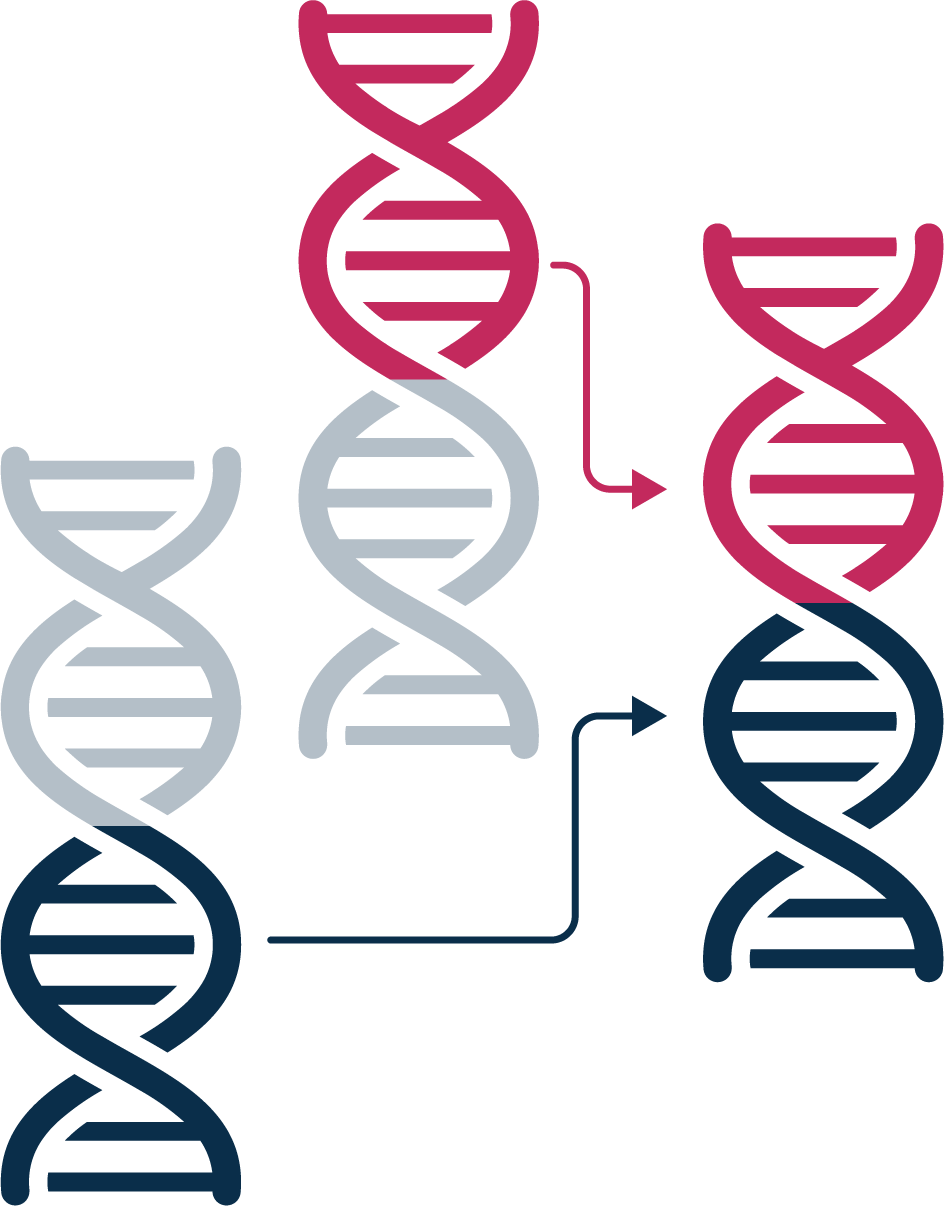
Gene fusions
Found across solid tumor types, such as lung, colorectal, skin and brain cancers. Our technology covers key fusion biomarkers, including NTRK and RET, and supports novel fusion detection.
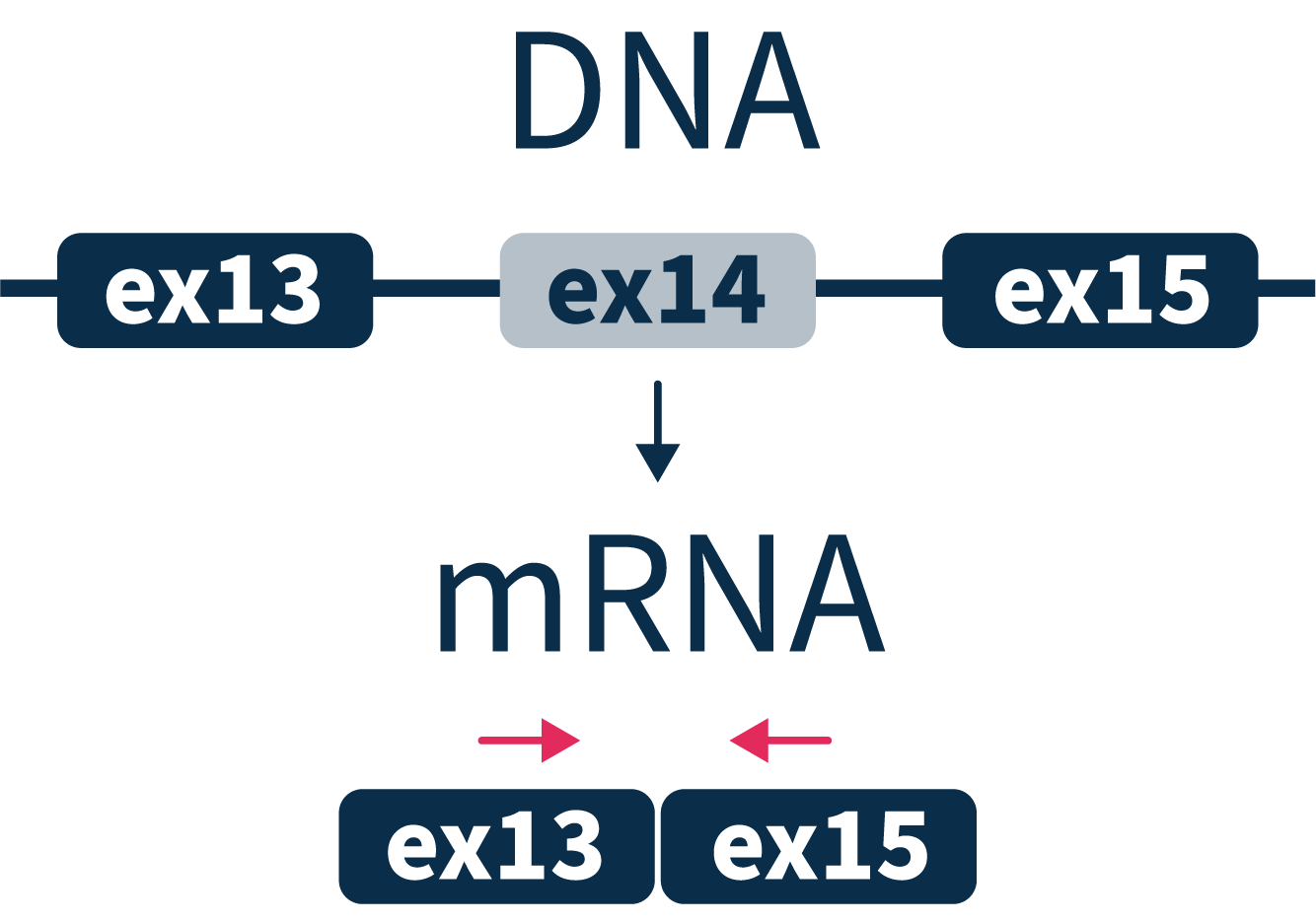
MET exon14 skipping
A rare oncogenic driver and therapeutic target for non-small cell lung carcinomas.

Gene amplification
Amplification has prognostic and diagnostic implications and is a mechanism of acquired drug resistance.

Microsatellite Instability (MSI)
A molecular fingerprint for defects in the mismatch repair pathway, associated with colorectal and other cancers.
Tumor Mutational Burden (TMB)
A prognostic biomarker that measures the quantity of mutations in tumor cells.

Genomic Integrity (GI)
Our deep learning-powered GI Index recognizes patterns of genomic instability associated with homologous recombination deficiency in high-grade serous ovarian cancer.
Streamlined variant assessment
Interpretation has been one of the most complex and time-consuming aspects of transforming genomic data into meaningful results. The SOPHiA DDM™ for Solid Tumors solutions are equipped with intuitive variant filters and automatic, artificial intelligence-enabled prioritization options that streamline and accelerate the interpretation workflow.
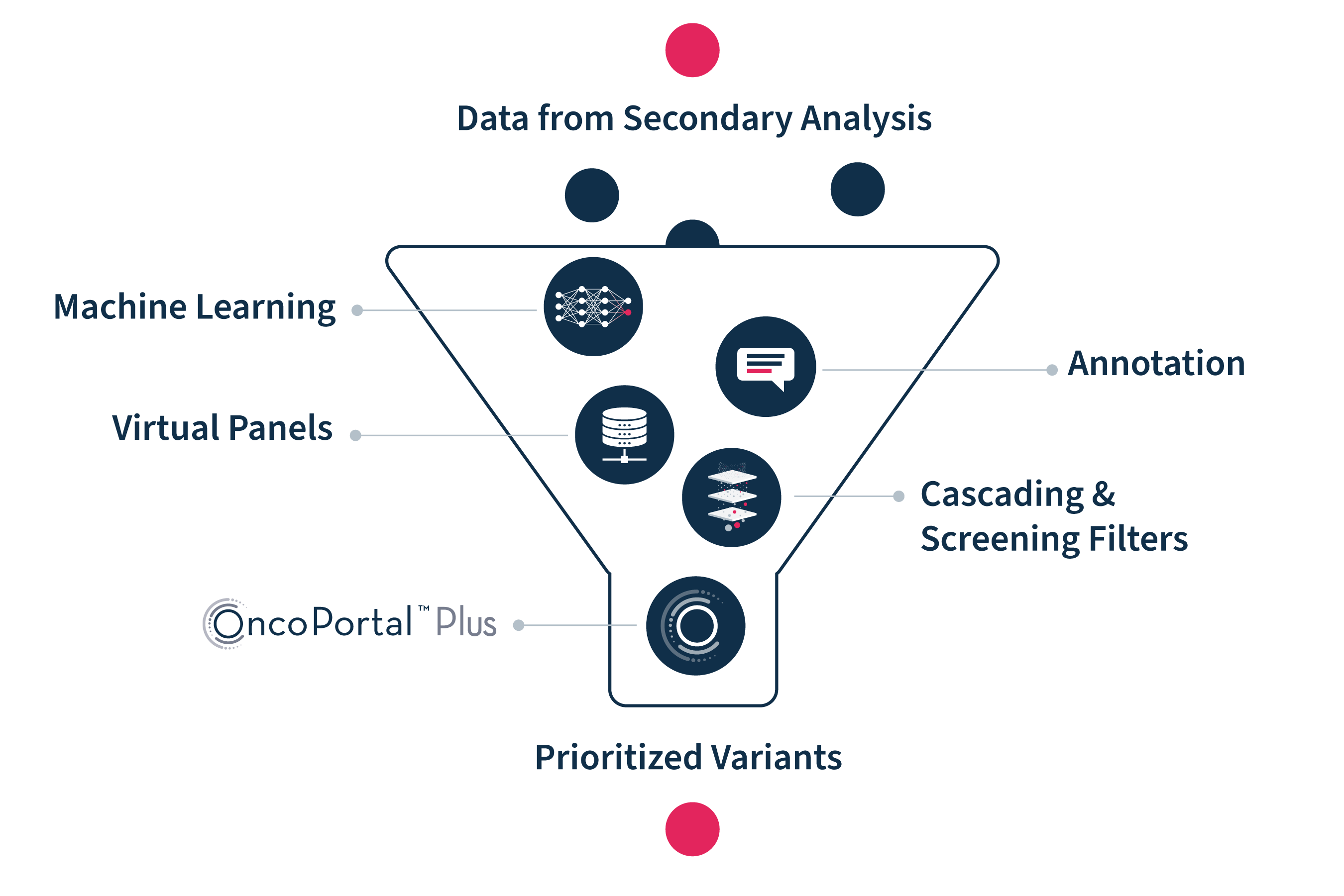
Illustrative representation of tertiary analysis workflow in SOPHiA DDM™.

Illustrative representation of the OncoPortal™ Plus functionality.
Advanced decision-making support
The OncoPortal™ Plus feature for SOPHiA DDM™ matches solid tumor molecular profiles with clinical associations and available clinical trials, leveraging expertly curated evidence powered by JAX-CKB. After interpretation, the flexible reporting tools enables you to prepare push-button, comprehensive reports that are customizable to your needs.
Discover our solutions
SOPHiA DDM™ HRD Solution
SOPHiA DDM™ for Liquid Biopsy

RNAtarget Technology by SOPHiA GENETICS
SOPHiA DDM™ for CGP
SOPHiA DDM™ Solid Tumor Solutions
SOPHiA DDM™ Homologous Recombination Solutions
CE-IVD products by SOPHiA GENETICS
