CE-IVD Oncology Applications by SOPHiA GENETICS™
Supporting fast and reliable precision diagnostics with CE-IVD applications powered by SOPHiA DDM™
We empower clinicians to make informed decisions
based on patient data
As genomic analyses become standard in both clinical and research settings, professionals are faced with the complex task of translating raw sequencing data into precision medicine approaches. Our in vitro diagnostic (IVD)-marked genomic applications offer end-to-end workflows that support accurate data analysis and reveal actionable insights, informing data-driven decisions and ultimately improving the quality of patient care.
Reporting made easy and accessible
- You want a quick turnaround time for your reports, but are still curious about how the data look on the inside?
- Discover our CE-IVD Oncology Applications powered by advanced analytical capabilities and helping clinicians and researchers to unlock valuable insight into tumor samples.
- Get your CE-IVD reports from our SOPHiA DDM™ Platform web/core which provides an intuitive workspace with a fast turnaround time.
- Use SOPHiA DDM™ core platform to receive additional clinical decision support (CDS) that allows further visualization, interpretation, and reporting of next-generation sequencing data.

Do you have questions on the new IVD Regulation?
Find your answers in our Frequently Asked Questions section
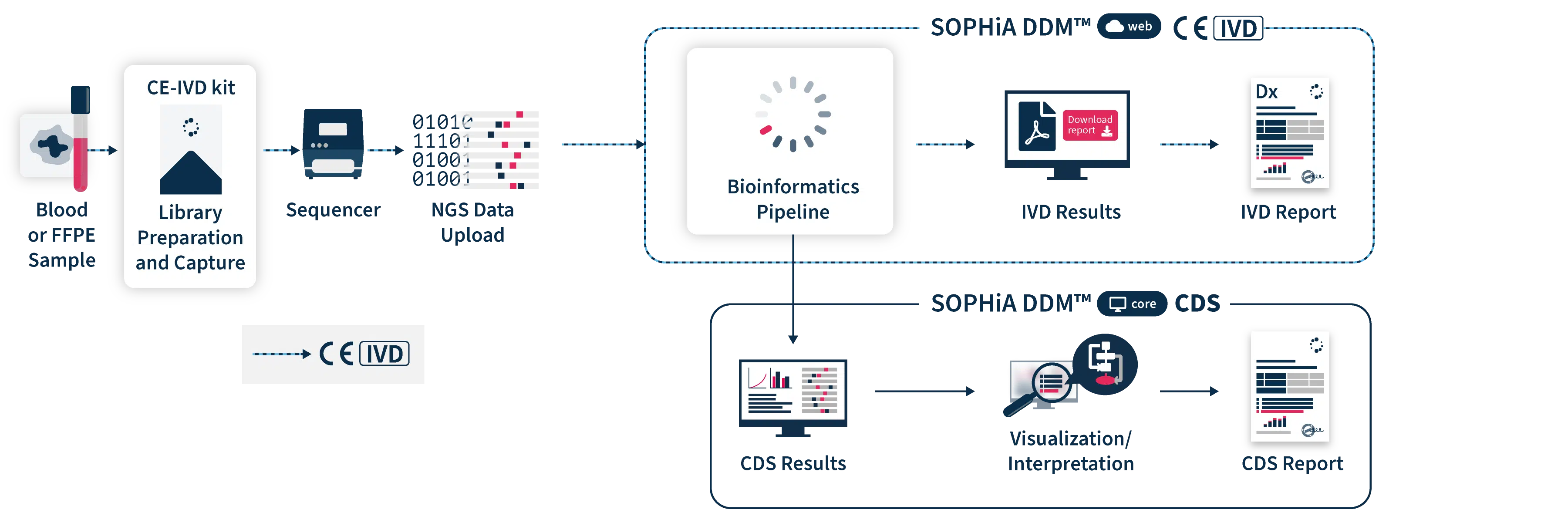
End-to-end workflow of of CE-IVD Oncology Applications by SOPHiA GENETICS™
(Tools and reports will vary depending on product.)
Unlock the power of precision oncology
Explore the benefits of our bundle solutions.
We enable one of the most diverse CE-IVD offerings in oncology – from sample to data to insight.
Detect SNVs, Indels and fusions confidently

Use clinical decision support to visualize and interpret your tumor data

Find patients who will get the most out of targeted therapy

Reduce time to patient benefit with the information you need on SOPHiA DDM™ Platform
Discover our CE-IVD applications
SOPHiA DDM™ Dx Homologous Recombination Deficiency (HRD) Solution
Support accurate detection of HRD status in ovarian cancer patients with our diagnostic application leveraging low-pass WGS to measure genomic scarring.
SOPHiA DDM™ Dx RNAtarget Oncology Solution (ROS)
Expect more from your RNA analyses with our diagnostic application providing reliable partner-agnostic detection of fusions in small lung cancer biopsy samples
SOPHiA DDM™ Dx Solid Tumor Solution (STS)
Benefit from advanced molecular profiling of solid tumors, including lung, colorectal, skin and brain, with our diagnostic application confidently detecting SNVs and Indels in relevant genes.
SOPHiA DDM™ Dx Myeloid Solution (MYS)
Expand the scope of myeloid neoplasms management with our diagnostic application providing advanced analytical performance for the detection of pertinent SNVs, Indels and FLT3-ITDs.
SOPHiA DDM™ Dx Hereditary Cancer Solution (HCS)
Confidently assess crucial variants associated with colorectal syndromes with our diagnostic application using capture-based target enrichment and tailored analytics to accurately detect key SNVs and Indels.
Frequently Asked Questions
What is the in vitro diagnostic regulation (IVDR)?
IVDR is the new In Vitro Diagnostic Regulation (EU) 2017/746 of the European Parliament and of the Council of April 5, 2017 on in vitro diagnostic medical devices, which went into effect on May 26, 2022 and repealing Directive 98/79/EC and Commission Decision 2010/227/EU.
How are IVD software and systems classified under IVDR?
Software/systems that drive a device or influence the use of a device shall fall within the same class as the device.
If the software/system is independent of any other device, it shall be classified in its own right depending on its application.
Can I still use my in-house assay and software?
In-house assays can be used until May 26, 2028 if the institution justifies that the target patient group’s specific needs cannot be met at the appropriate level of performance by an equivalent device available on the market.
Can I still use CE-IVD products that are on the market?
Yes. CE-IVD products registered under the IVDD can stay on the market in accordance with the timeline released by the European Commission for the product classification. Under the IVDR, SOPHiA GENETICS™ products are classified as Class C devices and can stay on the market until May 26, 2026.
What has changed with the new IVDR for genetic testing?
Classification and risk stratification of IVD products have changed and now also include genetic testing and companion diagnostics.
IVDR now includes software and in-house developed assays (IHAs). Both are subject to more vigorous control and are required for notified body approval.
More in-depth clinical data is required for manufacturers to demonstrate their safety and performance claims.
Notified body approval is now required for 80% of IVD products, compared to about 20% in the IVD directive.
Does the SOPHiA DDM™ Platform have CE-IVD?
When used with one of our 5 CE-IVD bundle solutions, SOPHiA DDM™ web platform is available as a CE-IVD product for In Vitro Diagnostic Use in Europe, and Turkey.
Which applications of SOPHiA DDM™ have CE-IVD certification?
SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution , SOPHiA DDM™ Dx RNAtarget Oncology Solution, SOPHiA DDM™ Dx Myeloid Solution, SOPHiA DDM™ Dx Hereditary Cancer Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products in Europe and Turkey.
SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are CE-IVD marked also in Israel.
Want to know more?
Get in touch with us.
Indels, insertions/deletions. ITDs, internal tandem duplications; SNVs, single nucleotide variants; WGS, whole genome sequencing