Pioneering Innovation, Streamlining Adoption
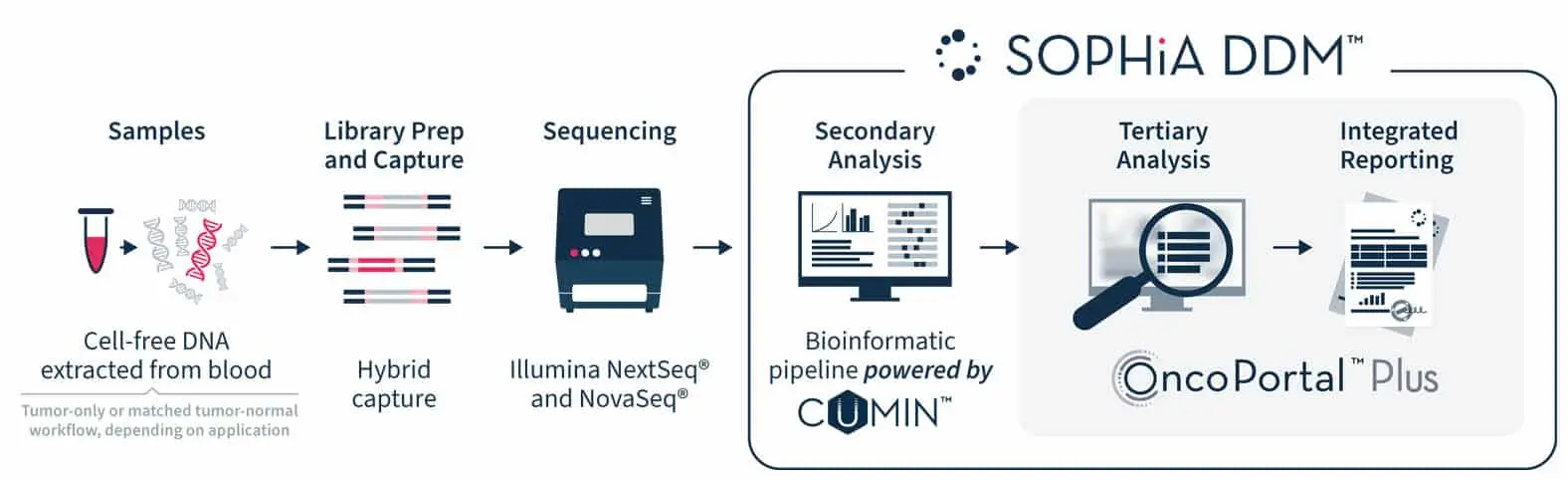
We are pushing the boundaries of today’s liquid biopsy capabilities. Bring liquid biopsy to your lab with our streamlined DNA-only NGS workflow, taking you from cell-free DNA sample to comprehensive report in record time. Powered by state-of-the-art proprietary algorithms, the SOPHiA DDMTM Platform reveals deep genomic insights from cell-free DNA, advancing your oncology research to new horizons.
Expand your capabilities with MSK-ACCESS® powered with SOPHiA DDM™
MSK-ACCESS® is a comprehensive liquid biopsy test developed by Memorial Sloan Kettering Cancer Center (MSK) that covers non-invasive cancer genomic profiling and disease monitoring1,2. It involves the deep sequencing of 146 key cancer-associated genes selected from MSK’s solid tumor genomic-profiling assay, MSK-IMPACT®.
Interested in adopting an in-house version of MSK-ACCESS®, enhanced with the advanced analytics of the SOPHiA DDM™ Platform?
SOPHiA GENETICS is collaborating with MSK to decentralize MSK-ACCESS® for liquid biopsy. By combining MSK’s clinical expertise in cancer genomics, the predictive algorithms of the SOPHiA DDMTM Platform, and the power of the global SOPHiA GENETICS community, the collaboration aims to expand access to precision cancer analysis capabilities worldwide.

CUMIN™: Elevating liquid biopsy analysis with breakthrough UMI technology
CUMIN™ is a proprietary unique molecular identifier (UMI) technology, designed to transform the way you analyze cell-free DNA samples with3,4:
Sensitive detection: detect variants with precision even at low frequency (down to 0.5% VAF), leveraging an alternative encoding approach.
Minimal input DNA: start from just 25 ng cell-free DNA.
Exceptional performance: experience comparable analytical performance to traditional UMIs.
Noise suppression: efficiently eliminate background noise for results you can trust.
Reliable quantification: obtain accurate molecule-based allele frequencies.
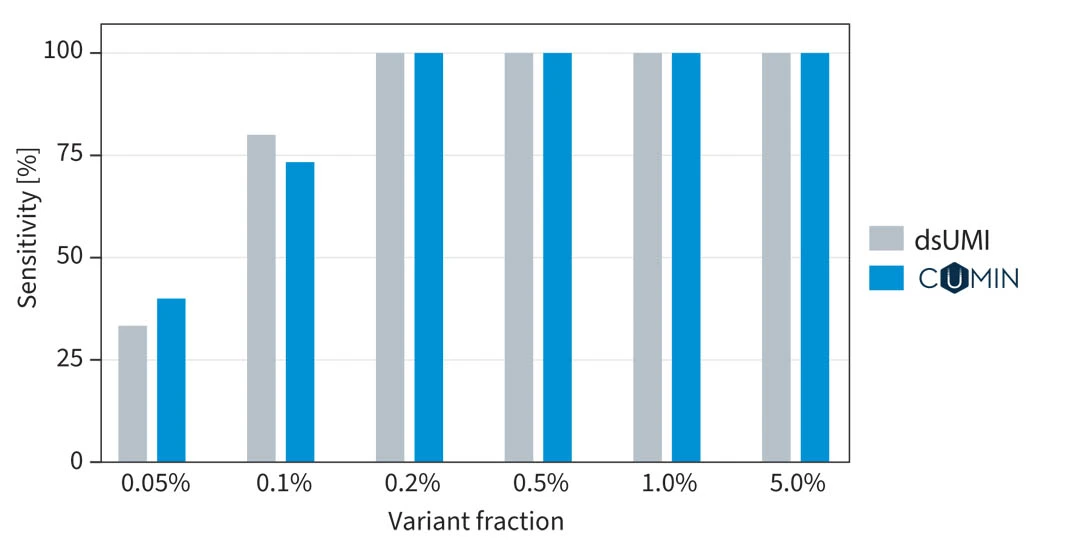
Variant calling performance when using proprietary SOPHiA GENETICS CUMIN™ technology compared with traditional dsUMIs (decoding by sequence)4.
Plug and tailor: one technology, infinite possibilities
Do you need a tailor-made solution to overcome your laboratory’s unique challenges? Get ready to embrace liquid biopsy.
Consult with our brilliant team of experts and embark on your liquid biopsy journey with:
An in-house wet lab workflow seamlessly integrated with the cloud-based analytics of the SOPHiA DDM™ Platform.
Customizable gene content, focusing exclusively on the most vital biomarkers for your lab.
Accurate detection of biologically actionable variants and biomarkers, including SNVs, Indels, fusions, and CNVs.
But that’s not all – if you’re already using SOPHiA DDM™ applications for solid tumor and homologous recombination repair (HRR) gene analysis, we’ve got you covered. You can add CUMIN™ technology to your workflow, maximizing the power of your current set-up.
You won’t be left alone. Enjoy comprehensive support at every step through the SOPHiA DDM™ MaxCare Program, making in-house adoption a breeze.
Choose a solution that fits your needs
| | MSK-ACCESS® powered with SOPHiA DDM™ | Adapt existing SOPHiA GENETICS solid tumor applications to liquid biopsya | Custom liquid biopsy applications tailored to your needs | |
|---|---|---|---|---|
| No. of genes | 146 genes curated by MSK experts | LBx STS (42 genes), LBx HRS (16 genes), ExtHRS (28 genes), and more | 20 genes and over | 80 genes and over |
| Cancer type | Across cancer types (NSCLC, prostate, pancreatic, biliary, bladder, breast and other cancers) | Solution-dependent | Solid tumors, focused on biological relevance | Solid tumors, broader research interests |
| Degree of customization | No | Yes | Yes | Yes |
| CHIP filtering | Yes | No | No | No |
| Sequencerb | Illumina NovaSeq™ 6000, NextSeq® 2000 | Illumina NovaSeq™, NextSeq® | ||
| Alterations | SNV/Indels, CNVs, gene fusions MSI (investigational)c | SNV/Indels Depending on solution, CNVs, gene fusions, and MSI may be availablec | ||
MSK-ACCESS® powered with SOPHiA DDM™
| | |
|---|---|
| No. of genes | 146 genes curated by MSK experts |
| Cancer type | Across cancer types (NSCLC, prostate, pancreatic, biliary, bladder, breast and other cancers) |
| Degree of customization | No |
| CHIP filtering | Yes |
| Sequencerb | Illumina NovaSeq™ 6000, NextSeq® 2000 |
| Alterations | SNV/Indels, CNVs, gene fusions MSI (investigational)c |
Adapt existing SOPHiA GENETICS solid tumor applications to liquid biopsyᵃ
| No. of genes | LBx STS (42 genes), LBx HRS (16 genes), ExtHRS (28 genes), and more |
| Cancer type | Solution-dependent |
| Degree of customization | Yes |
| CHIP filtering | No |
| Sequencerᵇ | Illumina NovaSeq™, NextSeq® |
| Alterations | SNV/Indels Depending on solution, CNVs, gene fusions, and MSI may be availablec |
Custom liquid biopsy applications tailored to your needs (≥20 genes)
| | |
|---|---|
| No. of genes | 20 genes and over |
| Cancer type | Solid tumors, focused on biological relevance |
| Degree of customization | Yes |
| CHIP filtering | No |
| Sequencerᵇ | Illumina NovaSeq™, NextSeq® |
| Alterations | SNV/Indels Depending on solution, CNVs, gene fusions, and MSI may be availablec |
Custom liquid biopsy applications tailored to your needs (≥80 genes)
| | |
|---|---|
| No. of genes | 80 genes and over |
| Cancer type | Solid tumors, broader research interests |
| Degree of customization | Yes |
| CHIP filtering | No |
| Sequencerᵇ | Illumina NovaSeq™, NextSeq® |
| Alterations | SNV/Indels Depending on solution, CNVs, gene fusions, and MSI may be availablec |
Resources
Press Releases
- SOPHiA GENETICS Enters New Collaboration with Memorial Sloan Kettering Cancer Center and AstraZeneca to Address Global Inequalities in Comprehensive Cancer Care
- SOPHiA GENETICS and MSK Collaborate to Combine Cancer Analysis Technology for Liquid Biopsies
- BioReference® Signs On as the First Laboratory to Use MSK-ACCESS® Powered with SOPHiA DDM™
- SOPHiA GENETICS Announces Expanded Suite of Liquid Biopsy Offerings
Get in touch to find out more
Our client services team is on hand to answer any questions or schedule your live demo.
References
- MSK. MSK-ACCESS®. Available at: https://www.mskcc.org/departments/division-solid-tumor-oncology/early-drug-development-service-phase-clinical-trials/precision-medicine-approach/msk-access;
- Brannon AR, et al. Nature Communications. 2021;12(1):3770.
- Bieler J, et al. J Transl Med. 2023;21(1):305.
- Kubik S, et al. Poster #105 presented at AGBT 2023 (6–9 February), Florida, USA.
Footnotes
a. Existing SOPHiA GENETICS somatic panels can be converted to liquid biopsy-equivalent applications as custom applications, as conversion will require a change in library preparation, addition of UMIs, and adaptation of bioinformatics pipeline. Existing probe design can be kept but may not be optimal for liquid biopsy application.
b. Multiplexing dependent on panel size and genomic alterations to be analyzed.
c. MSI status detection feasibility is under investigation and may not be available at first launch.
Abbreviations
CHIP, clonal hematopoiesis of indeterminate potential; CNV, copy number variations; ExtHRS, SOPHiA DDMTM Extended Homologous Recombination Solution; HRS, SOPHiA DDMTM Homologous Recombination Solution; Indels, insertions and deletions; LBx, liquid biopsy; MSI, microsatellite instability; SNV, single nucleotide variant; STS, SOPHiA DDMTM Solid Tumor Solution; VAF, variant allele frequency.