SOPHiA DDM™ Homologous Recombination Deficiency (HRD) Solution
Go beyond homologous recombination repair (HRR) mutation detection.
Expand identification of HRD positive ovarian cancers.
SOPHiA DDM™ HRD Solution combines information from germline and somatic HRR mutations (including BRCA1 and BRCA2) with a measure of genomic scarring. Accelerate and empower your clinical cancer research decisions with cost-effective, reliable, and timely in-house results.
Targeted sequencing of somatic and germline mutations in 28 HRR genes, including BRCA1 and BRCA2
High coverage of low-pass whole genome sequencing (WGS) data supports detection of genomic scars
Deep learning algorithm, GIInger™, reveals extent of genomic scarring through the Genomic Integrity Index
SOPHiA DDM™️ end-to-end workflow with high quality analytical and interpretation capabilities
Product Details
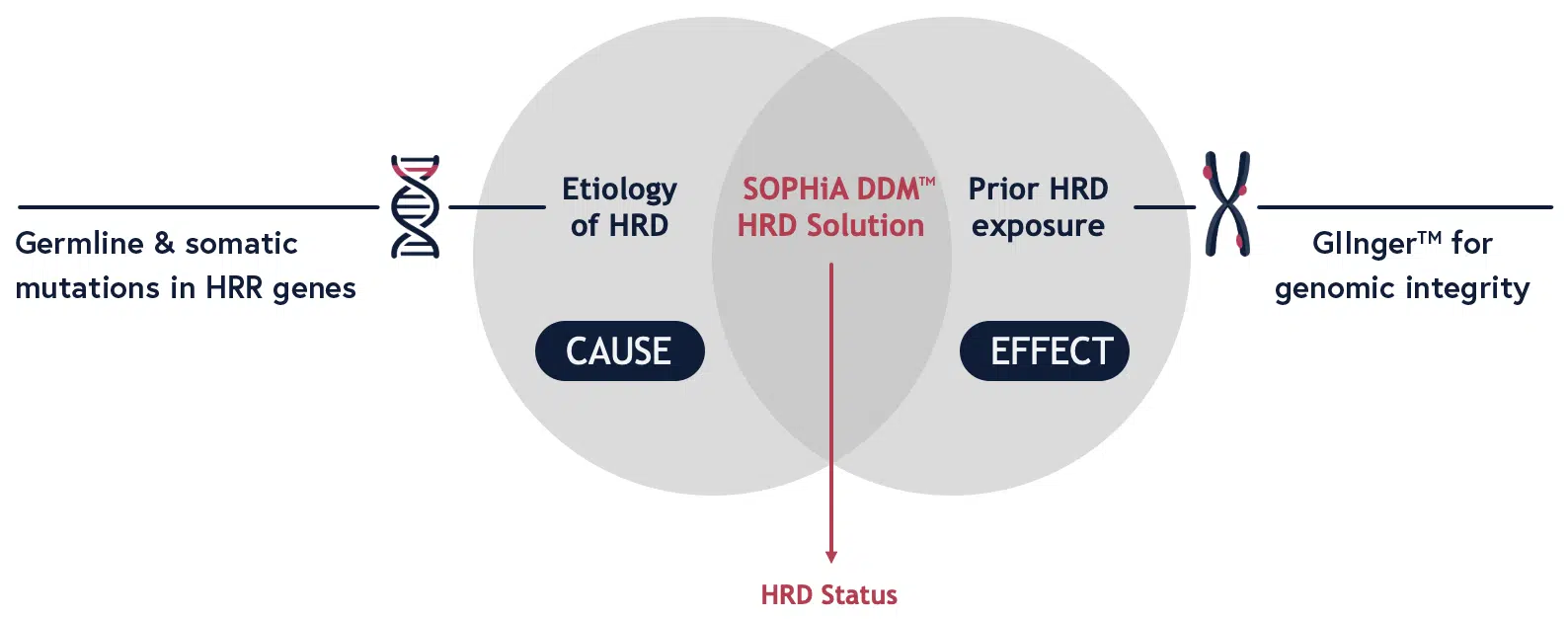
Detect HRD Cause and Effect in a Single Assay
SOPHiA DDM™ HRD Solution leverages target capture technology to isolate somatic and germline mutations in 28 HRR genes (including BRCA1 and BRCA2). This allows the user to identify pathogenic variants, such as SNPs and Indels, that could be potential causes of HRD.
To measure the impact of HRD, the SOPHiA DDM™ HRD Solution leverages low-pass WGS in conjunction with a convolutional neural network-based deep learning algorithm called GIInger™ to produce the Genomic Integrity Index. This score measures the extent of genomic scarring as a result of HRR mutations across the entire genome.
Combining the detections of HRR gene mutation and the Genomic Integrity Index in a single assay provides an accurate and sensitive method for determining HRD status of a tumor sample.
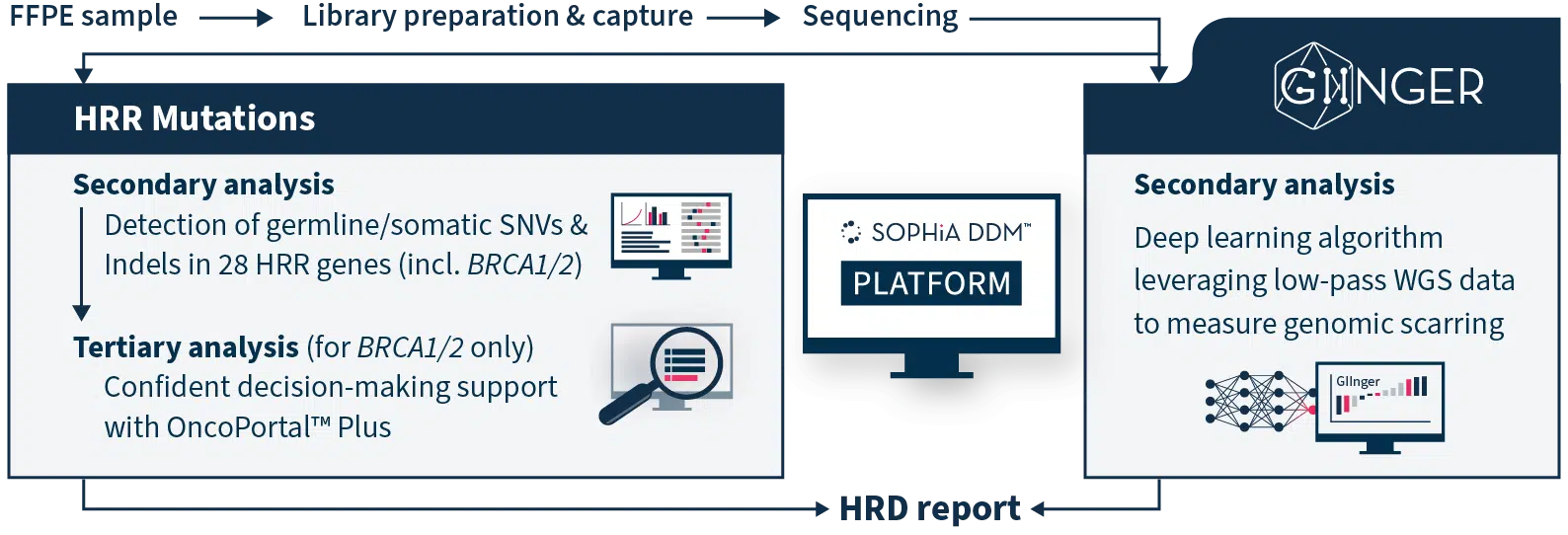
High Quality Analytics and Interpretation
SOPHiA DDM™ HRD Solution is a sample-to-report next generation sequencing (NGS)-based application, that combines an expertly designed wet lab kit with access to the analytical capabilities and interpretation-support functionalities of the SOPHiA DDM™ Platform. These solutions empower in-house expertise by providing high analytical performance and streamlined bioinformatics workflows with several intuitive features that accelerate variant assessment, interpretation, and reporting.
SOPHiA DDM™ in Action
Witness first-hand how the SOPHiA DDM™️ Platform streamlines the determination of tumor sample HRD status during this 3-minute tutorial video.
SOPHiA DDM™ HRD Solution Benefits
Easily and reliably assess HRD status with novel algorithms for HRD detection
Reduce turnaround time and costs with a comprehensive 2-in-1 solution
Build in-house expertise on HRD testing with a decentralized workflow, retaining ownership of all data
Identify mutations in 28 HRR genes and measure genomic scarring in a single solution
Specifications
| Parameters | Details |
|---|---|
| Diseases Covered | Ovarian cancer |
| Gene Symbols Covered | AKT1*, ATM, BARD1, BRCA1, BRCA2, BRIP1, CCNE1, CDK12, CHEK1, CHEK2, ESR1*, FANCA, FANCD2, FANCL, FGFR1*, FGFR2*, FGFR3*, MRE11, NBN, PALB2, PIK3CA*, PPP2R2A, PTEN, RAD51B, RAD51C, RAD51D, RAD54L, TP53. |
| Target Region Size | 88 kb |
| Key Biomarkers | BRCA1, BRCA2 |
| Sample Type | FFPE ovarian cancer tissue |
| Input Amount | 50 ng DNA per sample |
| Sequencer Compatibility | Illumina NextSeq® 550 |
| Library Preparation Time | 1.5 days |
| Analysis Time From FASTQ | 12 hours |
| Detected Variants |
|
* Hot spot coverage only
Resources
Related blogs
Contact us
Please fill out the form below to get in touch
Related products
SOPHiA DDM™ for Solid Tumors
From targeted to comprehensive genomic profiling, our solutions support healthcare professionals in their journey to analyze solid tumors.
SOPHiA DDM™ RNAtarget Technology
An advanced customizable RNA-based sequencing approach that offers a single workflow with confident novel fusion detection, while also providing ability to detect SNVs/Indels and expression changes.

SOPHiA DDM™ for CGP
SOPHiA DDM™ combines analytical performance with streamlined interpretation of complex genomic variants, (including SNVs, Indels, CNVs, fusions, MSI and TMB), in the context of comprehensive genomic profiling (CGP).
