SOPHiA DDM™ Solid Tumor Solutions
Profiling the cancer genome to optimize solid tumor management
Clinical oncology is increasingly adopting next-generation sequencing (NGS) in response to the growing number and type of biomarkers that require rapid assessment.
Targeted genomic profiling offers the ability to focus on a set of biomarkers known to be associated with a cancer. Compared to broader approaches, targeted gene sequencing reduces workflow complexity, avoids unnecessary sequencing costs, and minimizes the effort needed for variant interpretation and reporting.
Expertly designed panels, delivering a high on-target rate throughout the entire target regions
Ready-to-use target-enriched libraries generated in just 1.5 days for DNA and 6 hours for RNA
Customizable content to perfectly meet the unique needs of each research laboratory
High coverage uniformity, ensuring accurate variant detection (SNVs, Indels, CNV, MSI, and gene fusions) and unlocking the efficiency in multiplexing more samples per run
Streamline interpretation with the SOPHiA DDM™ Platform intuitive variant filters, algorithm-supported variant classification with OncoPortal™ Plus to obtain the latest scientific evidence on all the relevant variants
Have access to one of the largest networks of connected healthcare institutions within SOPHiA DDM™ Platform, to gain and share knowledge on relevant variants
Product Details
Unlock efficiency in multiplexing more samples per run
SOPHiA DDM™ Solid Tumor Solution (STS) and Solid Tumor Plus Solution (STS+) are NGS-based applications that combine expertly designed targeted panels with access to the analysis and interpretation functions of the SOPHiA DDM™ Platform. Both solutions enable accurate detection of SNVs, Indels and CNVs in addition to Microsatellite Instability (MSI) status in genes involved in solid tumors, such as lung, colorectal, skin, and brain cancers. SOPHiA DDM™ STS+ offers additional insights by also targeting 136 gene fusions, MET (exon 14 skipping), and EGFR (vIII exon skipping). Both solutions provide a streamlined sample-to-report workflow that greatly accelerates variant assessment, while ensuring confidence in your results.
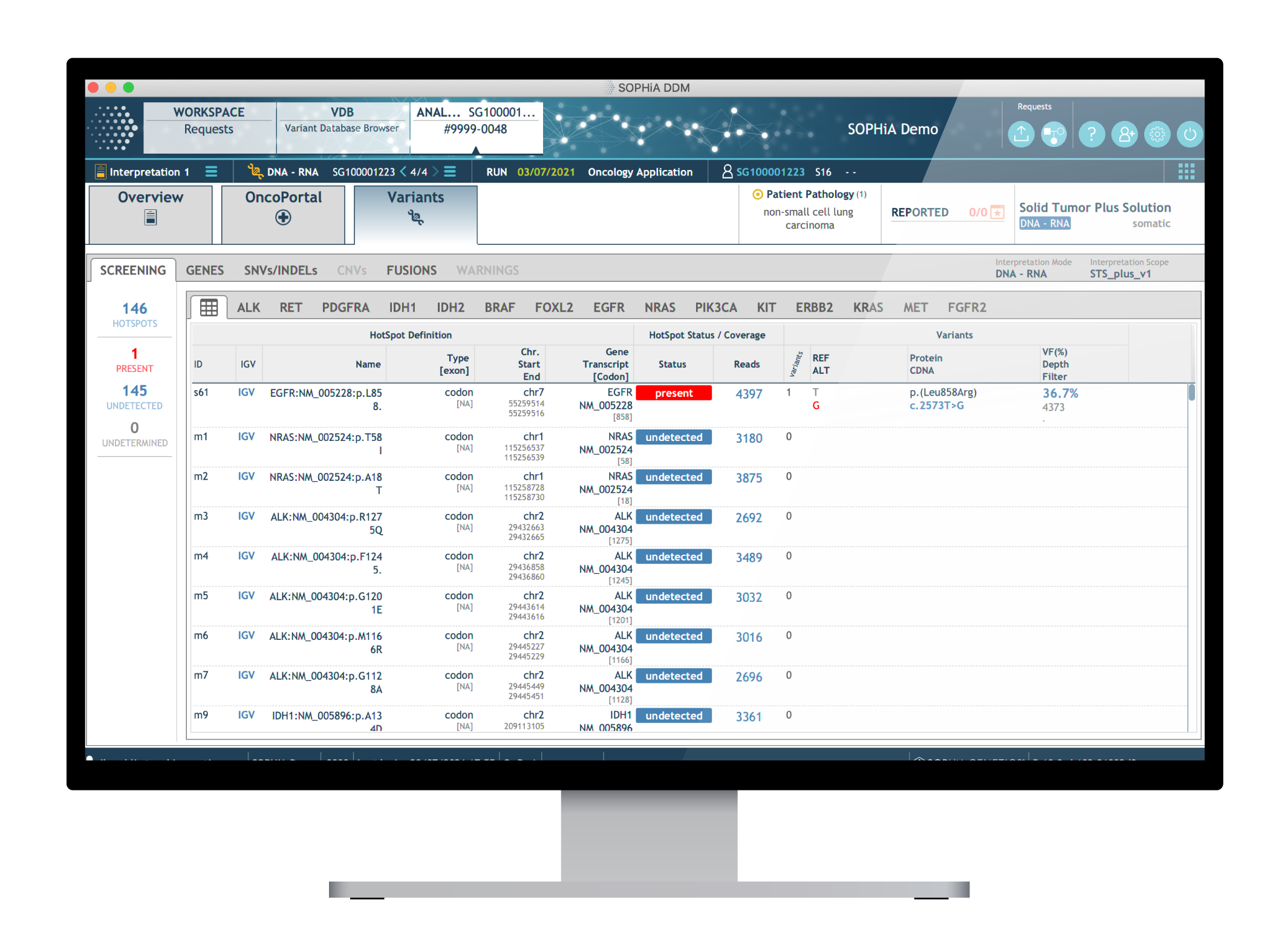
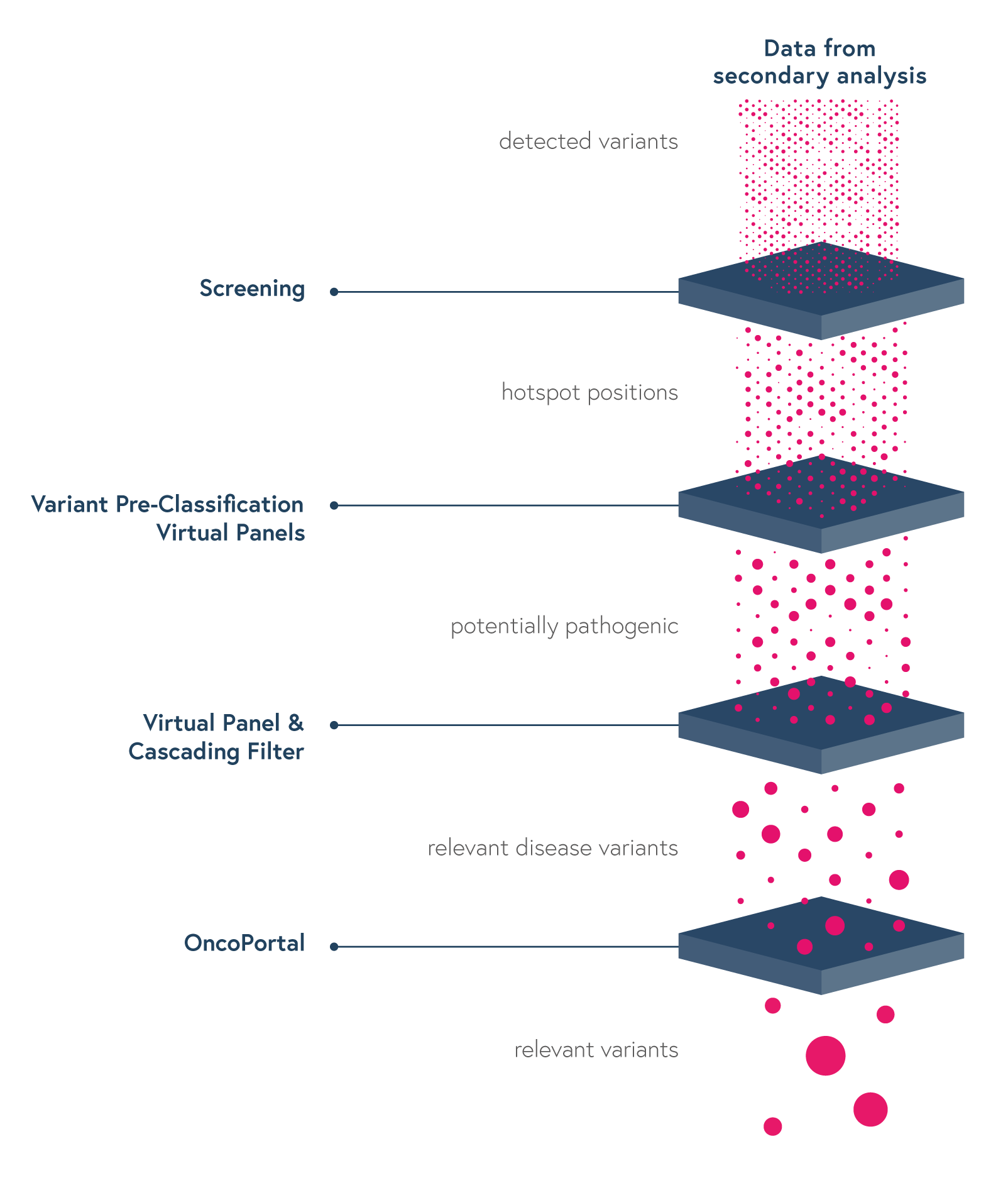
Dedicated features to ease variant interpretation
The SOPHiA DDM™ Platform features intuitive variant filters and prioritization options to streamline interpretation and help reduce turnaround time.
- Hotspot Screening to quickly pinpoint the relevant hotspots and provide a clear overview of the wild-type hotspot positions
- Variant Pre-Classification to facilitate assessment of variants’ pathogenicity
- Virtual Panels to limit interpretation to a subset of genes
- Cascading Filters to enable user-created custom filtering strategies for quicker identification of relevant variants
- OncoPortal™ Plus to support decisions based on the JAX-CKB, CAP, ASCO, AMP and other databases
Thoroughly assessed to provide confidence in your results
A six-center evaluation assessed SOPHiA DDM™ STS with more than 150 samples. The study confirmed reliable performance that can facilitate confident decision-making.
| Performance Metrics | Observed | Lower CI |
|---|---|---|
| Analytical Sensitivity | 98.77% | 93.31% |
| Analytical Precision | 100% | 96.25% |
| Analytical Specificity | 100% | 99.92% |
| Analytical Accuracy | 99.97% | 99.85% |
| Analytical Repeatability | 96.45% | 96.41% |
| Analytical Reproducibility | 89.13% | 89.05% |
| Coverage Uniformity | 98.7% | 92.5% |
Values have been calculated for SNVs and Indels from a total of 150 samples processed on MiSeq®.
Specifications
| Parameters | SOPHiA DDM™ STS | SOPHiA DDM™ STS+ |
|---|---|---|
| Diseases Covered | Lung, colorectal, skin, and brain cancers | Lung, colorectal, skin, and brain cancers |
| Gene Symbols Covered | 42 genes | DNA panel: 42 genes: RNA panel: 136 gene fusions, MET (exon 14 skipping), and EGFR (vIII exon skipping). |
| Target Region Size | 22 Kb | 22 Kb |
| Key Biomarkers | ALK, BRAF, EGFR, IDH1, IDH2, GNA11, GNAS, GNAQ, HIST1H3B, H3F3A, H3F3B, MET, KRAS, KIT, NRAS, MET, PIK3CA | Same biomarkers of SOPHiA DDM™ STS and fusion events involving genes such as ALK, BRAF, EGFR, FGFR1, FGFR2, FGFR3, NTRK1, NTKR3, PPARG, RET and ROS1 |
| Sample Type | FFPE, fresh-frozen tissue | FFPE, fresh-frozen tissue |
| Input Amount | 10 ng DNA minimum (50 ng recommended) | 10 ng DNA minimum (50 ng recommended), 100-200 ng RNA |
| Sequencer Compatibility |
|
|
| Library Preparation Time | 1.5 days for DNA | 1.5 days for DNA, 6 hours for RNA |
| Analysis Time From FASTQ | From 4 hours | From 4 hours |
| Detected Variants |
|
|
Resources
Contact us
Please fill out the form below to get in touch
Related products
SOPHiA DDM™ for Blood Cancers
Our future-proof end-to-end solutions allow detection and characterization of complex genomic variants associated with different blood cancers.
SOPHiA DDM™ for Solid Tumors
From targeted to comprehensive genomic profiling, our solutions support healthcare professionals in their journey to analyze solid tumors.
SOPHiA DDM™ for Hereditary Cancers
Our solutions help reduce time to confidently assess multiple types of challenging genetic variants that indicate predisposition to inherited cancers.