What is precision medicine?
Precision medicine, also known as personalized medicine, aims to enhance healthcare quality by tailoring treatments to each person’s unique genetic makeup, environment, and lifestyle. While a fully individualized approach to medicine is still a work in progress, the recognition that patient heterogeneity influences treatment effectiveness is not new1.
Historically, medicine has heavily relied on trial-and-error strategies for discovering, developing, and testing new treatments targeted at specific indications. This disease-centered approach resulted in predetermined standard therapies tailored to the “average patient.” While this one-size-fits-all approach has succeeded in many indications, it also carries significant drawbacks, particularly when dealing with complex diseases such as cancer or inherited disorders. In these cases, the risk of adverse side effects (e.g., toxicity) and reduced therapeutic response often result in poorer patient prognoses and quality of life1.
Precision medicine represents a patient-centric paradigm shift, acknowledging each individual’s uniqueness while using real-world data and advanced statistics to guide the discovery-to-treatment process. For instance, pharmacogenomics requires us to look at each patient genomic data individually and in the context of others, enabling stratification into cohorts for predicting treatment responses2.
Success in precision medicine hinges on the ability to derive meaningful insights from large patient datasets. Fostering data diversity has the potential to further advance progress in this area1,3.
Advancing healthcare through precision medicine
Recent technological advances have made precision medicine more accessible and impactful than ever before. Next-generation sequencing (NGS) has become more affordable, transforming it from a research-focused technology into a tangible clinical reality. This progress was further propelled by the widespread adoption of electronic health records (EHRs) and laboratory information management systems (LIMS), which not only facilitate population-scale research but also enable the use of clinical decision support tools for the delivery of targeted therapies to individual patients4.
The ability to identify genetic biomarkers and assess variant pathogenicity has grown significantly in the past decade. This has not only revolutionized patient diagnosis but also transformed drug development. A pivotal moment was the approval of imatinib by the FDA in 2001, the first small molecule targeted therapy for chronic myeloid leukemia (CML)5. By inhibiting the BCR-ABL fusion protein, imatinib was shown to effectively slow the progression of CML from chronic phase to blast crisis, making it the first of its kind.
This groundbreaking milestone paved the way for the approval of many other targeted therapies, such as gefitinib targeting EGFR alterations associated with NSCLC (2003) and trastuzumab for HER2-positive breast cancer (2006). The pace of new targeted drug approvals continues to accelerate year after year, heralding a promising era of precision medicine6.
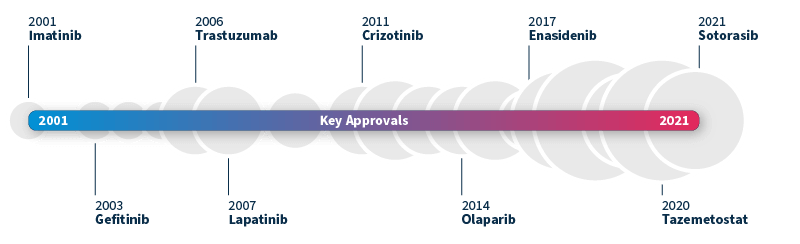
Timeline of FDA-approved targeted therapies in cancer. Grey bubbles represent the relative number of approvals per year. Data source: Waarts et al 2022.
Addressing the gaps in precision medicine
To achieve a truly personalized approach to medicine, the harmonization of translational and precision medicine is paramount. This coordination between early mechanism-based drug development and late-stage patient-centric approaches gives rise to an end-to-end biomarker-guided process, allowing us to optimize treatment strategies for patient cohorts right from the outset7.
Known as translational precision medicine, this emerging concept brings a fresh perspective to the translational gap, calling for a broader scope beyond a purely genetic-based definition of biomarkers and introducing a multimodal approach by taking into account a wider range of healthcare variables. To make this new concept a reality, significant technological progress is required in several key areas8:
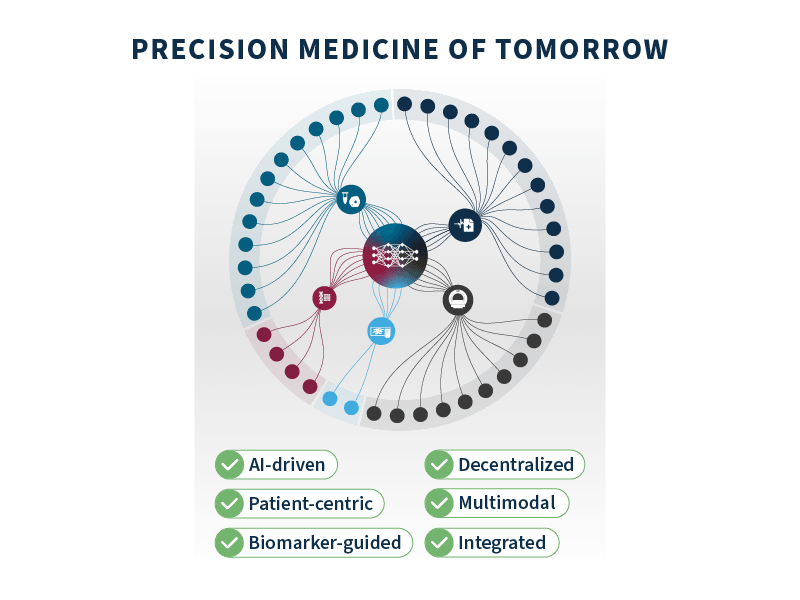
- Multimodal profiling: embraces a comprehensive analysis of various data modalities, including genetics, imaging, clinical, and biological data, among others.
- Data integration: most key data are currently siloed within systems, making integration of the utmost importance to unlock meaningful insights and correlations that can guide personalized treatment decisions.
- AI-driven analysis: harnessing the power of machine learning and neural networks allows for more efficient and precise data analysis, identifying signals and trends that could ultimately lead to improved outcomes.
- Biomarker-guided clinical trial design: centering clinical trials around specific biomarkers enables more efficient testing of new treatments and helps identify patient subgroups that derive more benefits from each targeted therapy.
- Patient-centric companion diagnostics (CDx): CDx play a pivotal role in matching patients with the most suitable treatments, placing each patient at the center of the diagnostic and treatment decision-making process.
By addressing these critical areas of advancement, we can pave the way for a future where each patient receives personalized treatments tailored to her or his unique needs and characteristics. The pursuit of translational precision medicine promises to revolutionize healthcare, offering improved patient outcomes and transforming the landscape of medical research and development.
SOPHiA GENETICS’ role in advancing precision medicine
Powered by proprietary algorithms and enriched with data from 750+ institutions*, the SOPHiA DDM™ Platform accelerates advances in the field of precision medicine. Its core mission centers on empowering clinical researchers across healthcare and biopharma spheres alike.
To learn more about SOPHiA DDM™ BioPharma Solutions for biomarker-centric discovery, development, and application deployment, visit our page.
References
- Kosorok MR and Laber EB. Precision medicine. Annu Rev Stat Appl. 2019;6:2363-286. doi: 10.1146/annurev-statistics-030718-105251
- Cecchin E and Stocco G. Pharmacogenomics and Personalized Medicine. Genes (Basel). 2020;11(6):679. doi: 10.3390/genes11060679
- Cooke Bailey JN, Bush WS, Crawford DC. Editorial: The importance of diversity in precision medicine research. Front Genet. 2020;11:875. doi: 10.3389/fgene.2020.00875
- Fountzilas E, Tsimberidou AM, Vo HH, et al. Clinical trial design in the era of precision medicine. Genome Med. 2022;14(1):101. doi: 10.1186/s13073-022-01102-1
- Cohen P, Cross D, Jänne PA. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat Rev Drug Discov. 2021;20(7):551-569. doi: 10.1038/s41573-021-00195-4
- Waarts MR, Stonestrom AJ, Park YC, et al. Targeting mutations in cancer. J Clin Invest. 2022;132(8):e154943. doi: 10.1172/JCI154943
- Hartl, D., de Luca, V., Kostikova, A. et al. Translational precision medicine: an industry perspective. J Transl Med. 2021;19:245. doi: 10.1186/s12967-021-02910-6
* The number of institutions represents active customers who have generated revenue through the SOPHiA DDM™ Platform usage or Alamut™ Visual Plus licenses as of September 30, 2022.