Case Study – Genomic Diagnostics, Australia
Genomic Diagnostics improve equity of access to hereditary cancer genomic analysis in Australia with the SOPHiA DDM™ Hereditary Cancer Solution v1.1.
Country
Australia
Research Challenge
Accessibility of hereditary cancer genomic analysis
Solution
SOPHiA DDM™ Hereditary Cancer Solution v1.1
Genomic Diagnostics, part of Healius Pathology, is an Australian national genomics laboratory that offers government-subsidized and patient-paid next-generation sequencing (NGS), covering reproductive genomics, cancer genomics, germline genomics, and paternity testing. They are a trusted testing partner for large teaching hospitals, federal and state government departments, and private practices.
“The SOPHiA DDM™ Hereditary Cancer workflow allows us to provide affordable, accessible, and quality genomic analysis for Australians”
Dr Mark Williams, PhD, FHGSA, Chief Scientist, Genomic Diagnostics
Challenge
To provide equity of access to hereditary cancer testing in Australia, by offering high quality, affordable, and efficient genomic analysis.
Approach
The Genomic Diagnostics laboratory had multiple criteria that were integral when designing and developing a new NGS hereditary cancer application. The assay had to include relevant gene content and be able to accurately and reliably detect SNVs, Indels, and copy number variations (CNVs). To meet national testing demands, the laboratory needed an affordable end-to-end NGS workflow that offered fast turnaround times (TATs). It was also essential that the application be scalable and user friendly, so that laboratory staff were able to use it consistently and in a reproducible manner.
Solution
SOPHiA GENETICS collaborated with Genomic Diagnostics to design the SOPHiA DDM™ Hereditary Cancer Solution v1.1 to include relevant genes covered by current literature, guidelines, and regulations. The assay was combined with SOPHiA GENETICS analytic and interpretation tools to create a complete end-to-end workflow. The fully integrated workflow comprises the SOPHiA DDM™ Hereditary Cancer Solution v1.1 powered by the SOPHiA DDM™ Platform and added interpretation Modules (Fig. 1). Genomic Diagnostics use the Alamut™ Visual Plus module of SOPHiA DDM™ for enhanced variant visualization, and Mastermind™ to provide comprehensive supportive evidence.
Figure 1. SOPHiA DDM™ Hereditary Cancer Solution v1.1 end-to-end workflow
Image sourced from presentation provided by Mark Williams, FHGSA, Chief Scientist, Genomic Diagnostics.
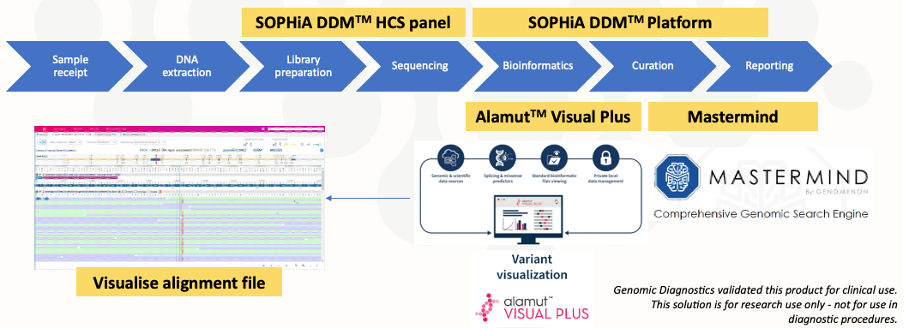
The workflow has successfully provided the Genomic Diagnostics laboratory with high-quality, consistent results with a full record of curation, while enabling visualization of BAM files for robust variant analysis. The SOPHiA GENETICS application is automated in the laboratory to minimize hands-on time and includes a secure bioinformatics pipeline that meets Australia’s privacy regulations. All staff in the high-throughput laboratory can effectively use the end-to-end application, even without prior bioinformatics expertise. The user-friendly software has a visual interface, and provides users with detailed QC metrics, annotation information, and helpful links to databases.
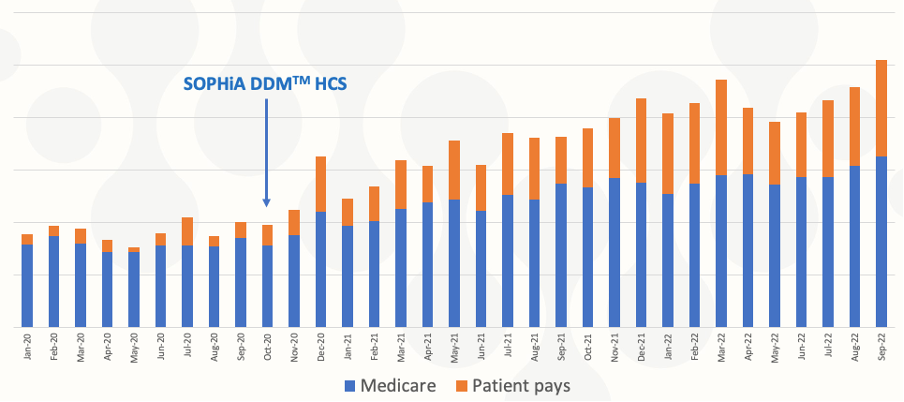
In conclusion, the SOPHiA DDM™ Hereditary Cancer Solution v1.1 accurately and reliably detected SNVs, Indels, and CNVs in samples analyzed by the Genomic Diagnostics laboratory. TATs stayed stable even with increasing testing volumes year-on-year, demonstrating the scalability of the end-to-end application. The integrated workflow and affordable costing allowed Genomic Diagnostics to increase testing volumes (Fig. 2), meeting their goal of facilitating equal access to hereditary cancer genomic analysis.
Figure 2. Increasing access to hereditary cancer genomic analysis over time
Chart sourced from presentation provided by Mark Williams, FHGSA, Chief Scientist, Genomic Diagnostics.
Watch the full story as told by Mark Williams at Genomic Diagnostics
Mark explains how implementing the SOPHiA DDM™ Hereditary Cancer NGS workflow enabled the laboratory to provide affordable and quality genomic analysis to strive for equity of access. He includes real examples of how SOPHiA DDM™ and Alamut™ Visual Plus were used to visualize BAM files and investigate variants such as CNVs, and shows metrics and genetic findings from 2 years of use.
Genomic Diagnostics validated this product for clinical use.
This solution is for research use only – not for use in diagnostic procedures.
Related Products
SOPHiA DDM™ for Hereditary Cancers
Confidently assess genetic variants predisposing to cancer
Alamut™ Visual Plus
For variant annotation, deep exploration, and enhanced visualization










