Menu
Menu
Interpreting genetic variants remains one of the most complex and resource-intensive steps in clinical genomic workflows. This technical note outlines how Alamut™ Visual Plus addresses these challenges by unifying essential annotations, prediction tools, and visualization features within a single, intuitive platform that integrates seamlessly into existing laboratory processes.
Read on for a practical overview of how Alamut™ Visual Plus streamlines and enhances variant interpretation workflows.
Dr. Gilles Millat, Laboratory of Molecular Cardiogenetics, Hospices Civils de Lyon, France.
For more than two decades, Dr. Millat has focused on the genetics of hereditary cardiac disorders at the University Hospital of Lyon. Originally trained in molecular biology, his work evolved from lysosomal storage disorders to inherited cardiac diseases, particularly cardiomyopathies and cardiac arrhythmias.
These disorders present important interpretive challenges:
Hypertrophic and dilated cardiomyopathies alone affect approximately 1 in 200-500 individuals. As demand for genetic testing grows, his laboratory expects to process ~5,000 cases in 2025, including 2,500 new probands, with volumes increasing 10–20% each year.
Dr. Millat’s laboratory has used SOPHiA DDM™ for more than a decade, now integrated as a central element in the analysis workflow. He highlights three key benefits:
“With just a few clicks, we can access everything needed to process a patient’s file from A to Z in only a few minutes.”
To date, nearly 15,000 patient cases have been analyzed, with approximately 700 variants per case, all systematically classified on a scale of 1-5.
In addition to the core platform, the SOPHiA DDM™ Peer Network enables some expert cardiogenetics labs across France to share variant interpretations. This collective expertise provides confirmation and confidence for complex cases.
Case example: Filamin C variant reclassification
A 70-year-old patient with hypertrophic cardiomyopathy presented with a rare FLNC variant. Although evidence supported pathogenicity, concerns about background noise led the Lyon team initially to classify it as a Variant of Uncertain Significance (VUS - Class 3).
Through the Cardio Peer Network, they found that colleagues in CHU Nantes had seen the same variant, also classified as a VUS, with a highly similar phenotype. Collaborative review allowed both teams to upgrade the classification to Class 4 (likely pathogenic).
This reclassification enabled relatives in both families to be offered presymptomatic testing, supporting earlier surveillance and informed management decisions.
“Without the Cardio Peer Network, we would have kept this variant as Class 3. Clearly, the Cardio Peer Network helped us.”
Together, SOPHiA DDM™ and the Cardio Peer Network have enabled scale, precision, and expertise sharing at CHU de Lyon, improving variant classification and informing care for patients and families affected by inherited cardiac pathologies.
Interested in SOPHiA DDM™ Peer Networks?
Connect with our team to learn how your laboratory can contribute to advancing variant classification in your field of expertise.
The opinions expressed during the video are those of the speaker and may not represent the opinions of SOPHiA GENETICS.
Any use of SOPHiA GENETICS products described in the video may not have been cleared or approved by Regulatory Authorities.
SOPHiA DDM™ Solutions are For Research Use Only unless otherwise specified.
For individuals living with undiagnosed rare conditions, the journey to answers is often long and filled with uncertainty. At SOPHiA GENETICS, we support partners around the world to help bring clarity through advanced genomic analysis. One such story comes from São Paulo, Brazil, where a woman’s decades-long search for answers finally came to an end thanks to the work of the Bioma Genetics Laboratory.
The patient, a 44-year-old woman, had lived her entire life struggling with unexplained symptoms. Her skin was unusually thin and stretchy, her joints overly flexible, her bones so fragile that even minor impacts caused fractures, and she had never been able to have children.
Despite years of consultations, her symptoms remained undiagnosed, leaving her without answers or effective treatment options.
Eventually, her doctor referred her to Bioma Genetics, with the hope of finding molecular evidence to support a suspected diagnosis.
Dr. Rafael Malagoli’s team at Bioma Genetics performed genetic testing using the SOPHiA DDM™ Whole Exome Solution v2 and identified two homozygous pathogenic mutations. These mutations confirmed the clinical suspicion of Ehlers-Danlos syndrome (EDS), a rare inherited connective tissue disorder.
For the patient, this diagnosis was life changing. She finally had a scientific explanation for the challenges she had faced her entire life - her skin and joint symptoms, her bone fragility, and her infertility. The emotional weight of decades of uncertainty was eased.
Empowered with this knowledge, she began connecting with others living with EDS. These new connections gave her access to community support, shared experiences, and practical advice on managing her condition. It also opened the door to personalized care and more informed decision-making.
So meaningful was the result that she personally visited the Bioma Genetics team to thank them. Her gratitude reflected not only the significance of receiving a diagnosis, but also the sense of agency and peace it finally brought her after decades of uncertainty.
This story underscores the transformative potential of genomic medicine, particularly when combined with the right tools, such as the SOPHiA DDM™ Platform, and the expertise of dedicated laboratories like Bioma Genetics. For clinicians and laboratories working with rare and inherited disorders, genomic analysis is more than a technical solution, it’s a path to answers that can change lives.
And for patients like this woman, it’s a reminder that understanding your genome can be the key to reclaiming your story.
SOPHiA DDM™ Whole Exome Solution v2 is for Research Use Only (RUO) and not for use in diagnostic procedures. Clinical interpretation and patient management decisions are the sole responsibility of qualified healthcare professionals. Patient case shared with permission and anonymized for educational purposes.
This Technical Note explores key considerations for PGx analysis, with a focus on the advantages of copy number variation (CNV) detection and next-generation sequencing (NGS) over traditional methodologies.
We introduce the SOPHiA DDM™ Community Pharmacogenomics Solution, expertly designed for rapid and accurate identification of PGx-related variants, enhancing precision medicine through advanced analytics.
At the 2025 American College of Medical Genetics and Genomics (ACMG) annual meeting in Los Angeles, we hosted a focused session on the role of exome and whole genome sequencing (WGS) in clinical and research settings. The goal was to spark conversation about available technologies, implementation challenges, and future strategies. Four expert speakers shared insights on clinical utility, followed by a lively audience Q&A.
This blog captures key takeaways from the event, including when and why broader testing is preferred over targeted panels, how to optimize virtual panels, and reimbursement realities. Whether you’re a lab director, clinician, or genetic counselor, these insights offer timely guidance.
Historically, targeted panels have been the cornerstone of many diagnostic workflows. However, as our understanding of gene-disease associations evolves, so too must our approach to testing.
Exome sequencing can adapt to the dynamic nature of clinical genetics. Take carrier screening, for example. As Mahmoud Aarabi, Medical Director of UPMC Cytogenetics Laboratories, explained, the list of genes recommended for autosomal recessive and X-linked carrier screening by ACMG1 is continuously updated based on new phenotypic and population data. Rather than continually revising panel content, exome sequencing provides a flexible, future-ready alternative. Phenotyping also plays a critical role. In prenatal testing, complete phenotyping can boost exome diagnostic yield by 7–10%.2
Jeanette McCarthy, Principal Consultant at Zifo, emphasized how labs can maximize efficiency using virtual panels (slice panels) to analyze exome data. With this approach, labs validate the wet lab component once and can then revise gene content as needed without extensive revalidation.
But designing virtual panels well requires careful forethought. She recommended selecting only genes with robust disease-gene validity, accounting for technically challenging targets (e.g., SMN1, PMS2), and avoiding copy number variation (CNV) analysis for genes that lack sufficient evidence of a loss-of-function mechanism. Additionally, genes should be excluded when the only relevant variant types cannot be reliably detected by exome sequencing - for example, including ATXN7 is unhelpful due to exomes’ inability to detect repeat expansions.
Exome sequencing consistently delivers higher diagnostic yield and cost savings compared to traditional approaches
Joe Jacher, Genomenon and trained genetic counselor, highlighted the clinical and economic case. “You can save $20,000 by skipping from microarray straight to exome,” he noted, citing peer-reviewed research.3 Indeed, the literature supports exomes/genomes as first- or second-tier tests for congenital anomalies or intellectual disability.4 For neurodevelopmental disorders (NDDs), exome sequencing outperforms chromosomal microarray analysis in both diagnostic yield and cost-effectiveness when used early.4,5
One of the largest challenges to broader adoption of exome and genome sequencing in clinical settings is insurance coverage. Despite proven utility, reimbursement remains inconsistent and often favors exome over genome sequencing, which is often restricted to research use.
In pediatric oncology, for example, current guidelines may still prioritize legacy tests like karyotyping and FISH over broader sequencing approaches, even when those legacy tests fall short of delivering a diagnosis.
To navigate this, some labs are adopting hybrid models. At Stanford Medicine, clinical panels are run on a genome backbone, enabling targeted reporting first, with the option for expanded analysis later if required. It also positions the lab for a future where broader genome analysis becomes more widely accepted and reimbursed.
If insurers continue to favor exomes, exome-on-genome workflows may be a practical interim solution to futureproof workflows and streamline reanalysis as new insights emerge.
WGS offers some clear technical advantages. It covers both coding and non-coding regions, provides more uniform coverage than exomes, and captures structural variants and repeat expansions with greater accuracy.
Jennefer Carter, Senior Genetic Counselor and part of the Stanford Undiagnosed Diseases Network (UDN), described how WGS delivered diagnoses in cases where exome sequencing would have failed. Among 283 Stanford UDN patients, WGS revealed diagnoses in cases involving CNVs/structural variants, repeat expansions, and non-coding variants – challenging variant types often missed by exomes.
Genome sequencing is where we’re headed - but exomes are the most practical, reimbursable, and clinically validated tool we have right now
Audience members agreed. One noted that "genome + RNASeq is the way forward," pointing to savings from eliminating multiple legacy tests. But RNASeq has its own limitations, such as with conditions affecting tissues where genes are not expressed in the blood.
Moreover, there were warnings of variability in commercial genome testing. Some labs restrict genome interpretation to just 10 bp into introns unless another variant prompts deeper review. Transparency and education are essential to ensure providers understand what their patients are receiving.
Despite historical limitations, some institutions are already shifting toward genome-first approaches. A genetic counselor from Children's Hospital Los Angeles noted that their team now defaults to WGS for most send-outs. Encouragingly, insurer coverage is improving, and third-party labs have been able to cover costs to build evidence for future reimbursement.
So, what can clinical labs and providers do today to prepare for an exome- and genome-enabled future?
While WGS promises broader insights, exome sequencing remains the most practical and reimbursable tool today. It balances diagnostic yield, cost, and flexibility, making it a strategic choice for many clinical settings.
As infrastructure, interpretation tools, and reimbursement models continue to evolve, WGS will play a growing role in routine care. But for now, optimizing the use of exomes while laying the groundwork for a genome-based future, offers the best of both worlds.
The discussion at ACMG 2025 made clear: the path to better patient outcomes lies in making high-quality genomic testing more accessible, informed, and actionable.
Visit our Rare Disorders page to learn more about SOPHiA DDM™ exome and genome solutions.
References
Please can you introduce yourself and share a bit about your role, institution, and the genetic services you provide?
I am a laboratory scientist working in the Molecular Diagnostics Laboratory at the National Children’s Hospital “Carlos Saenz-Herrera” in Costa Rica. Our laboratory serves as the national reference center for diagnosing genetic diseases in both children and adults. Additionally, we are part of the National Oncological Counseling Project, which has been operational since 2018. Within this project, we perform genetic analyses for adult participants across the country. I am part of the team responsible for conducting genetic tests, including whole exome sequencing and Sanger sequencing, as well as analyzing, interpreting, and reporting variants. Our laboratory collaborates closely with clinicians from various hospital specialties, holding monthly clinical meetings to discuss complex and challenging cases. This multidisciplinary approach helps improve diagnosis and patient management.
Which SOPHiA GENETICS applications do you currently use in your work?
Currently, we use the SOPHiA DDM™ Hereditary Cancer Solution v2.0, which includes 83 genes associated with hereditary cancer, and the SOPHiA DDM™ Whole Exome Solution. We rely on the SOPHiA DDM™ Platform for variant analysis.
What are the key benefits of using the SOPHiA DDM™ Platform in your lab?
Using the SOPHiA DDM™ Platform has significantly streamlined our workflow by reducing analysis time and optimizing variant interpretation. The platform provides easy access to multiple databases directly within its interface, which simplifies data contextualization.
One of the most valued features is the ability to build our own custom database. This functionality allows us to perform intra-sample comparisons (within the same run), inter-sample comparisons (across all runs over time), and inter-laboratory comparisons using SOPHiA DDM™'s tools. This is essential for validating findings and ensuring consistency in our analyses.
Having a custom database is particularly beneficial because public databases often lack adequate information about Latin American populations, especially Costa Rican populations. By maintaining our own database, we can track allele frequencies specific to our population and analyze potential founder effects or population-specific genetic behaviors. This capability not only facilitates trend analysis over time but also enhances our ability to identify patterns and anomalies in genetic data. Such insights are invaluable for developing more precise and personalized clinical strategies tailored to our population.
Your recent research using the SOPHiA DDM™ Hereditary Cancer Solution v2.0 in Costa Rica identified a prominent founder variant in the BRCA2 gene. Can you share more about this discovery and its significance?As part of our involvement in the National Oncological Counseling Project, we have analyzed approximately 1500 probands and 2300 relatives since 2018. Among these families, around 800 have hereditary breast/ovarian cancer syndrome. Notably, we observed that 43% of families diagnosed with breast/ovarian cancer have a pathogenic variant in BRCA2. Of these families with BRCA2 variants, 61% carry the c.9235delG variant.
This variant does not fall within regions typically associated with breast or ovarian cancer risk. Interestingly, it has also been identified in other types of cancer within Costa Rica, such as pancreatic and prostate cancer. These findings suggest a unique genetic architecture for breast and ovarian cancer in the Costa Rican population.
The discovery of this founder variant highlights the importance of understanding population-specific genetic markers to improve diagnostics.
How has your experience been working with the SOPHiA GENETICS team?
Our collaboration with SOPHiA GENETICS has been highly positive. The team is responsive and supportive, providing valuable guidance on how to maximize the platform’s functionalities for our specific needs.
Looking ahead, what excites you most about the future of genetic testing at National Children’s Hospital “Carlos Saenz-Herrera”?
Looking ahead, we are excited about the possibilities of integrating cutting-edge technologies into our laboratory workflows.
For instance:
- Optical Genome Mapping: This technology has enormous potential to revolutionize structural variant detection by providing high-resolution insights into chromosomal abnormalities.
- Somatic Sequencing: Expanding into somatic sequencing will enable us to analyze tumor-specific mutations more comprehensively.
These advancements will further enhance our ability to provide robust diagnostic solutions and personalized treatment strategies for patients across Costa Rica.
Elexandra Barboza-Arguedas and her team are a great example of how dedicated experts can combine technology and local knowledge to make a real difference. We’re proud to support their work as they continue to expand genetic testing in Costa Rica, uncovering insights that not only advance clinical insights today but help shape a more personalized, inclusive future for healthcare.
Click through to learn more about SOPHiA DDM™ exome and hereditary cancer solutions.
We are glad to host Dr. Davide Martorana, Senior Molecular Geneticist at the Medical Genetic Lab of the University-Hospital of Parma in Italy, who shared with us his institute’s experience with the adoption of the New Generation SOPHiA DDMTM Platform.
-Hello, Davide, thank you for joining us today for this spotlight! Could you please briefly introduce yourself and describe your role, institution, and the type of services you offer?
-Sure. My name is Davide Martorana, and I am a Senior Molecular Geneticist at the Medical Genetic Lab – a lab at the University Hospital of Parma in Italy.
As a Biologist, I am part of the clinical interpretation team for focused panels and clinical whole exome sequencing for subjects with a suggestive phenotype; after the genetic test, we provide reports with results interpretation.
-Could you please share with us which of the SOPHiA GENETICS applications and services are you currently using?
-We use two different SOPHiA GENETICS solutions, the Nephropathy Solutions (NES) and Hereditary Cancer Solutions (HCS), analysed with SOPHiA DDMTM
-What are the biggest benefits you see in the New Generation SOPHiA DDMTM Platform in terms of new features/ capabilities and user experience?
-First of all, I want to state that we are very happy and satisfied with this evolution of the SOPHiA DDMTM Platform. The feature I prefer is the personalization of the information flow about genetic variants; in fact, I am sure every analyst has a preferred workflow when evaluating a single variant; for example, I like to see immediately the population frequency, both in our single center and for all SOPHiA GENETICS customers; then, the ACMG-AMP classification with specified criteria.
In my opinion, this fact is very important, because when you must manage a lot of variants, it is important to focus fast on a few but essential info, then if you want there is the possibility to access several other additional info; in particular, I appreciate the extensive link-outs to other databases, which are very accurate and useful.
-Thank you for your kind words. Could you please share an example where the SOPHiA DDMTM Platform streamlined your laboratory workflows and supported your clinical research efforts?
-In a specific family, after genetic counseling of a twenty-week-old pregnant woman with a clinical diagnosis of Polycystic Kidney disease 1 - never investigated at a genetic level - we were asked to analyze the woman and the fetus with the Nephropathy solution kit.
After the analysis on the SOPHiA DDMTM Platform, we were able to detect the causative mutation in PKD1 gene in the mother but not in the fetus. The analyses were performed in just three days after receiving the biological samples, which was a very fast turnaround.
After that analysis, we realized the true potential of having an NGS kit coupled with software with fast, reliable, and accurate results, and this is just one simple example.
-It is great to see how our solutions are impacting your real-life clinical research and streamlining your decision-making when it matters the most. On the implementation side, how was your experience with the Setup program for SOPHiA DDMTM in terms of easiness, time spent on validation, and time to routine?
-In our experience, the Setup program for SOPHiA DDMTM was very fast and easy. In fact, after the training with SOPHiA customer support, we spent just a working for validating the entire process and personalizing the information flow before introducing it in the routine analysis. This is very important and efficient for the users.
-And how does your experience with the SOPHiA GENETICS customer support look like?
-I am extremely satisfied with SOPHiA GENETICS customer support because they are efficient and proactive, answering all our queries and addressing our issues in a very short time and with great competence and kindness. I would like to thank you on behalf of the entire team for that.
We would like to warmly thank Dr. Davide Martorana for joining us in this customer spotlight.
Are you interested in exploring how the New Generation SOPHiA DDM Platform can revolutionize your workflows? Check out our recent blog post here!
The term « SOPHIA » used by the speaker refers to SOPHiA GENETICS and its products. The opinions expressed during this presentation are these of the speaker and may not represent the opinions of SOPHiA GENETICS. SOPHiA GENETICS does not provide support in the validation of custom products for clinical use. SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution is available as a CE-IVD product for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
Discover how Henry Ford Health streamlined their genetic testing by moving from three separate assays to a single, powerful exome-based solution. Learn how the SOPHiA DDM™ Exome Solution v3 consolidated their workflows for hereditary cancer, cystic fibrosis, and pharmacogenetics, offering broad gene coverage with a compact assay footprint. Dive into the full story to see how this innovative approach enabled detection of SNVs, Indels, and CNVs in one comprehensive assay, simplifying their germline testing processes.
Explore this infographic summary to discover the key findings from Janin et al.’s publication on next-generation sequencing (NGS) for the diagnosis of inherited cardiac diseases.

Click here to read the full publication.
Janin A, Januel L, Cazeneuve C, Delinière A, Chevalier P, Millat G. Molecular Diagnosis of Inherited Cardiac Diseases in the Era of Next-Generation Sequencing: A Single Center's Experience Over 5 Years. Mol Diagn Ther. 2021 May;25(3):373-385.
SOPHiA GENETICS products are for research use only – not for use in diagnostic procedures.
The HGVS nomenclature guidelines are used worldwide for genetic variant interpretation but can seem complicated and difficult to understand and apply. That is why we have created this beginner’s guide to mutation nomenclature using the HGVS recommendations, with clear visual examples that break down the process into bitesize pieces.
1. What is HGVS nomenclature?
2. How to read mutation nomenclature: Breaking down the variant description
2.1 Reference sequence e.g., NM
2.2 Description of variant e.g., c.4375C>T
2.3 Predicted consequence e.g., p.(Arg1459*)
3. The 3 prime rule for mutation
4. Final thoughts and helpful tool
The Human Genome Variation Society (HGVS) nomenclature standard was developed to prevent the misinterpretation of variants in DNA, RNA, and protein sequences. The HGVS nomenclature standard is used worldwide, especially in clinical diagnostics, and is authorized by the Human Genome Organisation (HUGO).1,2
HGVS General Terminology Recommendations1
| X Do not use | ✔️ Recommended terminology |
| Mutation or polymorphism | Variant, change, allelic variant Can be used for cancer tissue: Mutation load and tumor mutation burden |
| Pathogenic | Affects function, disease-associated, phenotype-associated |
HGVS follow recognized standards for the nomenclature of DNA and RNA nucleotides, the genetic code, amino acid descriptions, and cytogenetic band position in chromosomes.3
The HGVS recommendations for mutation nomenclature state that the format of a complete variant description should first include the reference sequence, followed by the variant description, and then the predicted consequence in parentheses. For example, NM-004006.2:c.4375C>T p.(Arg1459*) (Figure 1).
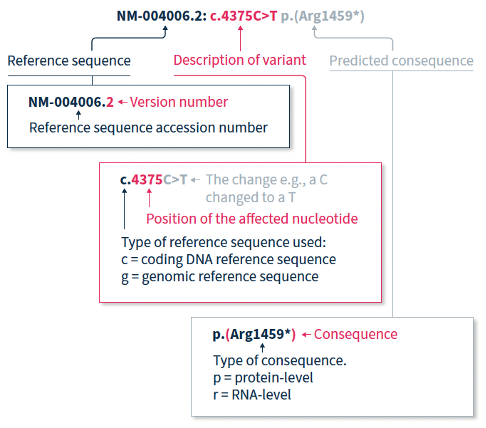
The HGVS nomenclature recommendations for sequence variants state that a complete variant description should begin with the reference sequence.1 The reference sequence accession number begins with a two-letter abbreviation (explained in Table 1), followed by a multi-digit number, and finally a version number.
Table 1. Meaning of the two-letter abbreviation at the beginning of a reference sequence accession number.
| Abbreviation | Reference sequence based on a: |
| NC | Chromosome |
| NG | Gene or genomic region |
| LRG | Locus Reference Genomic sequence: Gene or genomic region, used in a diagnostic setting |
| NM | Protein-coding RNA (mRNA) |
| NR | Non-protein-coding RNA |
| NP | Protein (amino acid) sequence |
The variant description begins by depicting the type of reference sequence used (c = coding DNA sequence, g = genomic reference sequence). When a protein-coding reference sequence is used (c), the nucleotide numbering begins with a 1, which represents the first position in the protein-coding region (the A of the translation-initiating ATG), and ends at the last position of the stop codon. Thus, if you divide the position number by 3, you can identify the affected amino acid in the protein sequence e.g., using the same example as above, 4375/3 = 1459, indicating that the predicted consequence affects amino acid 1459, which is an arginine. Different variants are indicated using different notations (explained in Table 2).
Table 2. HGVS notation and examples for the most common types of mutations2
| Notation | Example | Explanation |
| > | c.4375C>T | Substitution of the C nucleotide at position c.4375 with a T |
| del | c.4375_4379del or c.4375_4379delCGATT | Nucleotides from position c.4375 to c.4379 deleted |
| dup | c.4375_4385dup or c.4375_4385dupCGATTATTCCA | Nucleotides from position c.4375 to c.4385 duplicated |
| ins | c.4375_4376insACCT | ACCT inserted between positions c.4375 and c.4376 |
| delins | c.4375_4376delinsACTT or c.4375_4376delCGinsAGTT | Nucleotides from position c.4375 to c.4376 (CG) are deleted and replaced by ACTT |
When only DNA has been analyzed, the RNA- and protein-level consequences of the variant can only be predicted, and should thus be reported in parentheses e.g., p.(Arg1459*) is the predicted effect at protein level (p) for the example described above.
For all variant descriptions using HGVS nomenclature, the nucleotide at the most 3’ position of the variation in the reference sequence is arbitrarily assigned to have changed (see how to apply this rule in Figure 2).4 The exception is for deletions/duplications around exon junctions for which shifting the variant 3’ would place it in the next exon.5
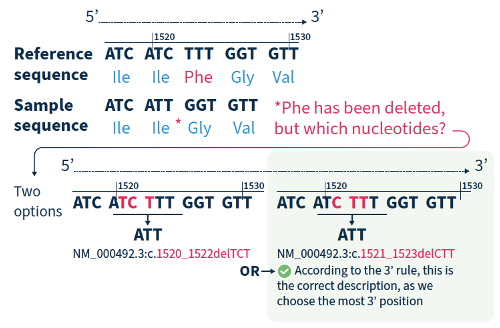
Although the HGVS recommendations can be difficult to understand and might take a bit of getting used to, if you break them down and refer to the examples in this guide, you are on the road to success!
If you want to accelerate your variant annotation and interpretation, Alamut™ Visual Plus is a comprehensive, full genome browser for efficient and user-friendly variant interpretation. The software accelerates the complex and time-consuming assessment of variants thanks to its user-friendly interface and integrated features for variant annotation and analysis.
Find out how Alamut™ Visual Plus applies the HGVS nomenclature recommendations to ensure that variant annotation follows the universally applied standards for variant analysis, interpretation, and reporting in our dedicated Technical Note.
Alamut™️ Visual Plus is for Research Use Only. Not for use in diagnostic procedures.
References
SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.