Menu
Menu
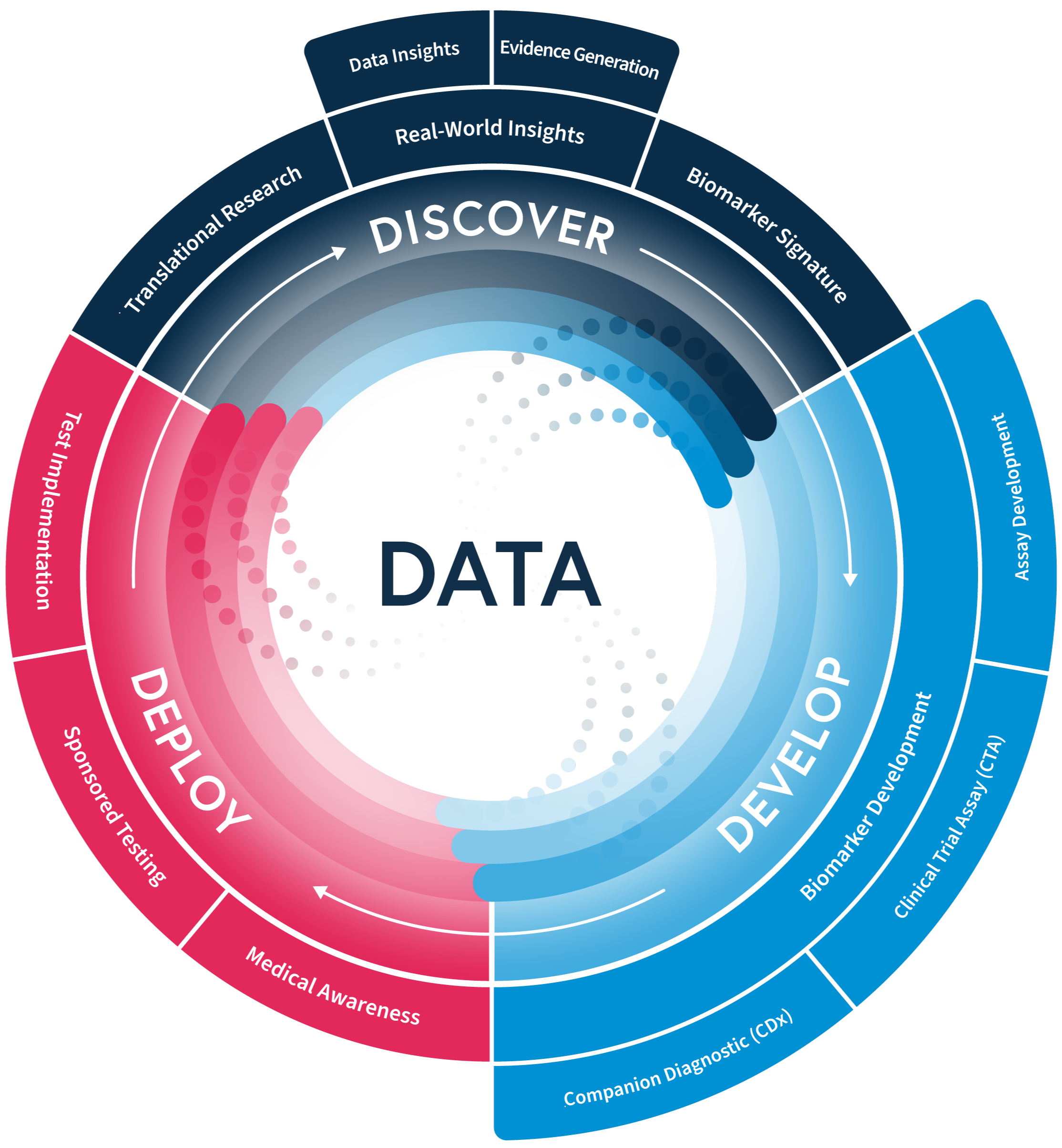
Rely on our implementation expertise to enable localized testing globally to precision therapies.

Myriad Genetics
A.D.A.M. Innovations
Precision for Medicine
We have joined forces with Myriad Genetics in a first-of-its-kind companion diagnostics (CDx) partnership model. This innovative hybrid approach combines Myriad’s centralized CDx and regulatory expertise with SOPHiA GENETICS’ decentralized, data-driven platform. Together, we enable the co-development of clinical trial assays and companion diagnostics, streamlined regulatory paths, faster execution, and scalable worldwide access to precision diagnostics and therapies.
We partnered with A.D.A.M. Innovations to democratize liquid biopsy testing and CDx commercialization in Japan, creating a unique value proposition to support pharma initiatives in this key market. Uniting A.D.A.M.’s local laboratory, regulatory expertise and market access with the SOPHiA DDM™ Platform, this alliance enables faster and cost-efficient NGS testing, advances pharma go-to-market strategies and maximizes access to precision therapies.
Precision for Medicine and SOPHiA GENETICS forged a strategic alliance that merges the strengths of each company, combining wet lab and translational expertise with advanced analytics. With this flexible model, we aim to develop elegant and limber clinical trial solutions that allow to derive useful molecular signatures from complex data, meeting pharma-specific needs, while advancing precision medicine through a data-driven collaboration.

We are in an era of rapidly evolving and increasingly complex rare diseases and cancers that demand an innovative and targeted approach.
SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.